
Read About the Heroes of RhoGAM
Order “Good Blood” by Julian Guthrie to learn about the story of how RhoGAM was invented.
Book


Plasma for RhoGAM is collected from dedicated KEDPLASMA centers before being manufactured. Every step of the production process for RhoGAM is owned by Kedrion Biopharma and takes place in the U.S.1,2
RhoGAM is made from human plasma. Products made from human blood may carry a risk of transmitting infectious agents (eg, viruses, the variant Creutzfeldt-Jakob disease [vCJD] and, theoretically, the Creutzfeldt-Jakob disease [CJD] agent)1
The human plasma that makes up RhoGAM undergoes a rigorous safety protocol, including donor selection and fractionation processes, a viral filtration step, and a viral inactivation process.1

COVID-19 is a highly contagious coronavirus infection spread by respiratory droplets. It is caused by SARS-CoV-2, a single-strand enveloped RNA virus.3
The viral filtration and viral inactivation processes remove and inactivate enveloped viruses similar to COVID-19.1
For more information on the donor process, please see the full Prescribing Information.

James Harrison was discovered by Australian scientists to have the anti-D antibodies needed for RhoGAM. Over his lifetime, he generously gave over 1,000 donations, a gift that has helped protect more than 2.4 million babies.6
RhoGAM owes its life-saving impact to the generosity of plasma donors. Discover more about their crucial contributions.
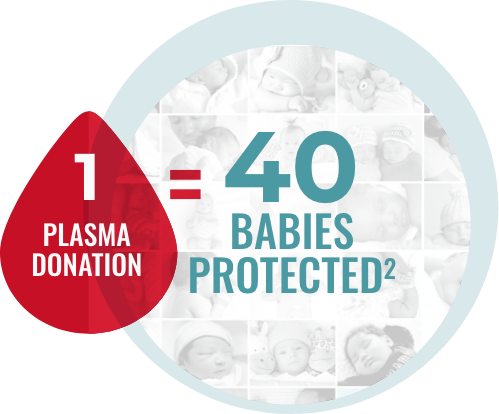
RhoGAM is made with human plasma provided by generous donors. Plasma is collected at designated KEDPLASMA facilities. Once accepted in the program after careful screening, donors can make up to 8 donations a month.1,2
Watch to catch a glimpse of life at Somerset Laboratory, one of RhoGAM’s plasma collection centers.

Order “Good Blood” by Julian Guthrie to learn about the story of how RhoGAM was invented.
Book
WIRhE is a non-profit organization dedicated to eliminating Rh disease worldwide.
Why RhoGAM
If you or someone you know is Rh-negative, see how you could help others by becoming an anti-D donor.
Articles