Life stories begin
with RhoGAM
The original Rh-negative breakthrough that continues to protect generations of babies1-4
RhoGAM Ultra-filtered PLUS (Rh0[D]) Immune Globulin (Human) is a prescription medicine given by intramuscular injection that is used to prevent Rh sensitization in Rh-negative expectant mothers.5
RhoGAM may help protect your Rh-negative pregnant patients from Rh sensitization. During pregnancy, Rh sensitization of the mother may lead to hemolytic disease of the fetus and newborn (HDFN) in future pregnancies.5
When to Administer RhoGAM
Routine Rh sensitization prevention5
If the father or the baby is not conclusively shown to be Rh-negative, RhoGAM should be given to an Rh-negative mother in the following clinical situations to prevent Rh immunization:
- At 26 to 28 weeks of pregnancy
- Within 72 hours of delivery of an Rh-positive baby
Other times of prevention5
- Maternal or fetal bleeding during pregnancy from certain conditions
- Actual or threatened pregnancy loss at any stage
- Ectopic pregnancy
- Amniocentesis
- Chorionic villus sampling (CVS)
- Manipulative procedures
- Other obstetrical trauma
A 300 µg syringe neutralizes up to 15 mL Rh-positive red blood cells (RBCs)—the equivalent of 30 mL fetal whole blood5
RhoGAM is intended for maternal use only and should not be administered to the newborn infant.
Additional doses of RhoGAM are indicated when the patient has been exposed to more than 15 mL of Rh-positive red blood cells. This may be determined by use of qualitative or quantitative tests for fetal maternal hemorrhage.
For complete dosing and administration information, click here for the RhoGAM Full Prescribing Information.
Committed to User Safety
Features you can count on1,5


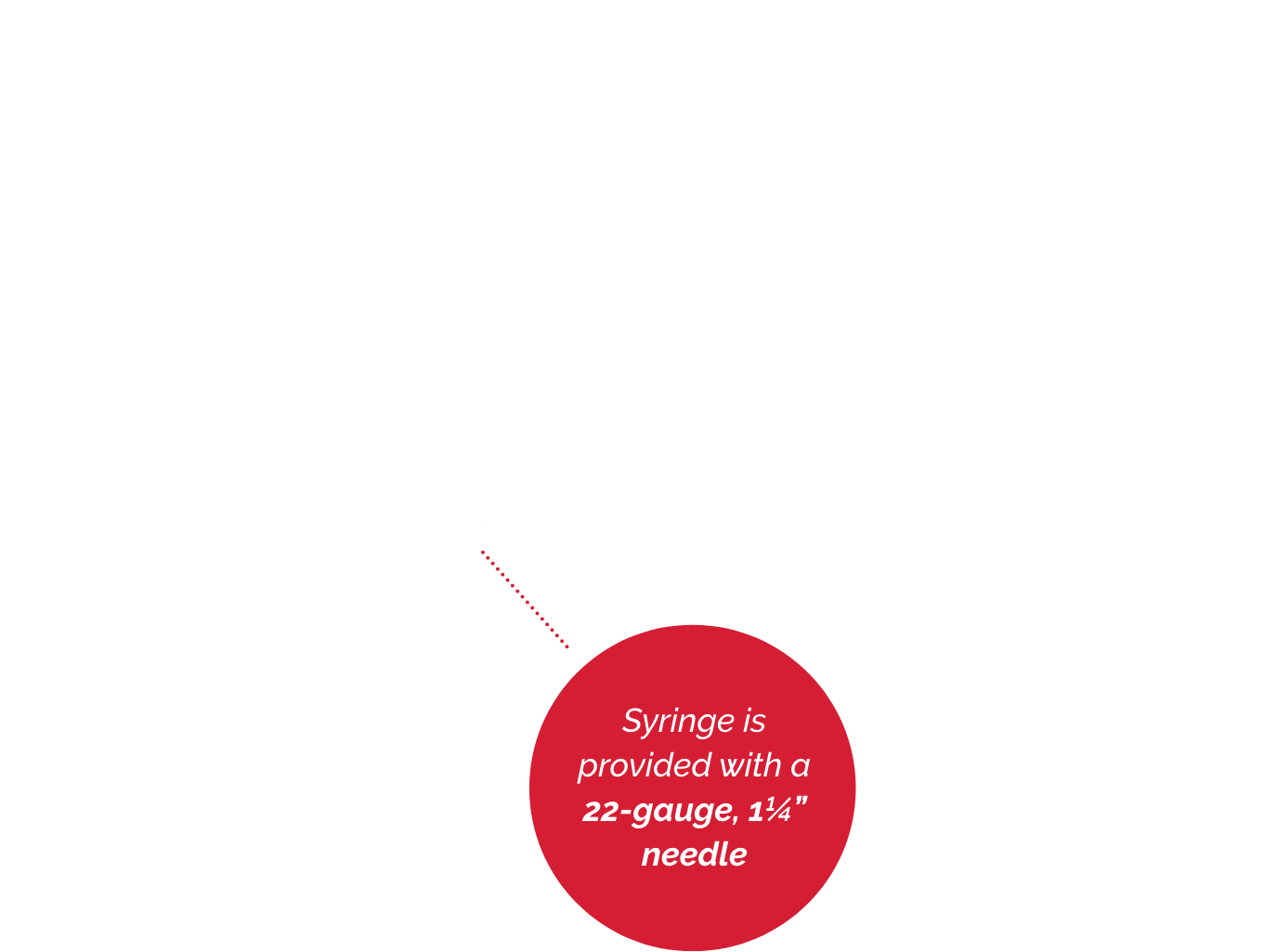

No temperature restrictions
Can be administered right out of the refrigerator
No light protection required
1 mL fill volume of 0.5 to 1.2 mL range
Each single-dose prefilled syringe contains 300 µg (1500 IU) of Rh0(D) Immune Globulin (Human)